by Brianna Crandall — June 12, 2020 — Quest Diagnostics, a provider of diagnostic information services nationwide and internationally, recently announced a suite of both on-site and at-home testing services to help enable organizations to screen their workforce for illness as buildings reopen amidst the ongoing COVID-19 pandemic.
Return to Work services
Quest Diagnostics launched a new suite of “Return to Work” services built around large-scale workforce COVID-19 testing. The services are designed to help organizations access and act on COVID-19 laboratory insights in order to foster safer workplace environments for their employees as they return to the workplace.
The Quest Diagnostics Return to Work services feature access to COVID-19 diagnostic and antibody testing and related capabilities, from event staffing to digital results reporting.
COVID-19 laboratory testing principally involves molecular diagnostics to detect viral RNA during active disease and serology testing to identify antibodies produced by the body following exposure to the virus. Both types of tests provide insights into disease risk for individuals and populations. Quest is committed to high-quality COVID-19 testing and analysis, and uses tests that have received U.S. Food and Drug Administration (FDA) Emergency Use Authorization (EUA) as well as met the company’s rigorous quality criteria.
Quest Diagnostics provides employee population health and workplace drug-testing services, serving thousands of employers across the United States. The company’s services range from lab and biometric screening to behavioral and mental health interventions to telemedicine and flu clinics.
To accommodate expected demand, Quest Diagnostics is scaling up its COVID-19 lab operations. The company expects to have capacity to perform approximately 150,000 molecular diagnostic tests a day by the end of June, compared to approximately 80,000 of these tests a day currently. The company also has capacity to perform approximately 200,000 antibody tests a day.
Modular approach tailored to different workforces
Jay G. Wohlgemuth, M.D., senior vice president and chief medical officer, and head of Quest’s Employer Population Health business, stated:
Employers of all kinds are trying to put programs in place to help protect their employees and customers as we return to work over the coming months. To be more effective, these programs will need to use high-quality laboratory-based testing with software applications and analytics along with implementation of worksite safety programs. Every workforce has different testing needs depending on the local environment, the type of work, the work environment and interactions with customers for the job category. Our Return to Work solution can be tailored to each organization’s unique requirements.
Among the modular features of the new service suite are:
- Event hosting that includes on-site temperature checks and respiratory and blood specimen collection by trained Quest Diagnostics staff. (Diagnostic testing is performed on respiratory specimens and antibody testing is performed on blood specimens.)
- Access to a variety of specimen collection options, ranging from respiratory specimen collection for diagnostic testing to use of the company’s 2,200 Patient Service Centers for blood draws for COVID-19 antibody testing.
- IT solutions that include secure online questionnaires to help direct participants to the appropriate testing (diagnostic or antibody) based on factors such as symptoms and exposure to sick individuals, and easy-to-understand results reporting.
- Access to physician ordering, oversight and telemedicine services.
- Data integrated with contact tracing and infection control software applications in use by employers.
- Influenza vaccination services are also provided and will ultimately incorporate SARS CoV-2 vaccines against the coronavirus that causes COVID-19, when available.
Quest Diagnostics is prioritizing availability of the testing services for employers with workforces critical to pandemic response, such as organizations that employ healthcare workers, first responders and other essential workers.
Self-collection Kit for COVID-19
Quest Diagnostics also announced that it has received Emergency Use Authorization (EUA) from the FDA for the Quest Diagnostics Self-collection Kit for COVID-19. The self-collection kit is for individuals to self-collect a nasal specimen at home or in a healthcare setting when determined to be appropriate by a healthcare provider.
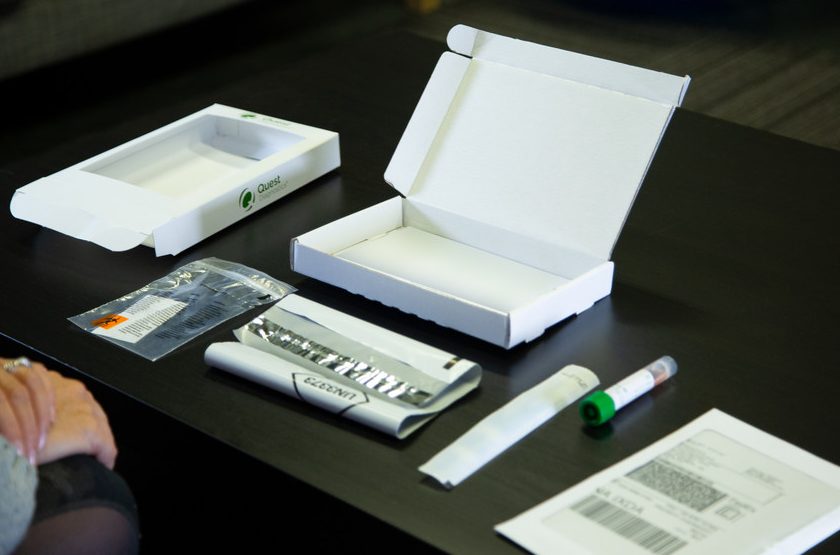
Consumer-friendly self-collection kits enable at-home testing; specimens are shipped overnight via FedEx at room temperature. Image courtesy Quest
The self-collection kit allows an individual to swab the front part of the nostril and may be used on children (supervised by an adult) as well as adults. Specimens are shipped overnight via FedEx at room temperature (without a frozen cold pack).
Specimens collected using the kit may be tested with the Quest Diagnostics SARS-CoV-2 RT-PCR test that received an Emergency Use Authorization in March. RT-PCR testing aids in diagnosing infection with SARS-CoV-2, the virus that causes COVID-19.
Steve Rusckowski, chairman, CEO and president, Quest Diagnostics, pointed out:
COVID-19 molecular diagnostic testing has been constrained partly by limited supplies of swabs and trained healthcare professionals to do the specimen collection. The self-collection kit enables an individual to self-collect at home, and the process is far less invasive and uncomfortable than many traditional methods.
Quest shared data with FDA that indicate that the self-collection kit, validated with real-world studies, offers a consumer-friendly approach to high-quality diagnostic testing for COVID-19. Quest Diagnostics already tested specimens using a similar collection method in real-world settings in drive-thru and other on-site COVID-19 testing sites across the United States.

The self-collection kit allows an individual to swab their nostril on their own. Image courtesy Quest
The self-collection kit allows the specimen to be collected at home and without the need to directly involve a healthcare professional to perform or observe the collection.
Key features of the new kit include:
- Self-collection by individuals, at home, with a consumer-friendly nasal swab approach
- Overnight shipping to the individual and back to Quest Diagnostics with FedEx, leveraging their extensive logistics network
- Specimens shipped at room temperature, which eliminates the need for ice packs
- Availability for children less than 18 years of age (with adult supervision)
- Results reporting through the myQuest patient portal and mobile app
- Test data reported by Quest Diagnostics to the relevant departments of health as required
The company plans to make the self-collection kits available through several channels, including for healthcare providers for patient care and healthcare workers as well as for states and organizations for return-to-work testing programs. Over time, the kits may also be made available to other employers as well as for individual users of the company’s QuestDirect consumer-initiated platform. The company will prioritize healthcare workers, first responders, law enforcement personnel and others critical to pandemic response to ensure they have timely access to the kit.
The company expects to have more than a half million kits available by the end of June, with plans to make additional kits available on an ongoing basis.
The Quest Diagnostics molecular test and self-collection kit are authorized only for the detection of nucleic acid from SARS-CoV-2, not for any other viruses or pathogens, and only for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostic tests for detection and/or diagnosis of COVID-19.
Quest Diagnostics says it is at the forefront of the response to the COVID-19 pandemic, working to broaden access to laboratory insights to help us all lead healthier lives. The company provides COVID-19 testing services under the Public Readiness and Emergency Preparedness Act. Quest provides data on COVID-19 testing to various federal and state public health authorities, including the US Centers for Disease Control and Prevention (CDC), and participates in studies with government and private institutions, aiding COVID-19 public health response and research.




