by Allan Browning and David Berry — Glycol-water mixtures are commonly used in facilities to provide freeze protection in HVAC closed loop heating and cooling systems. In addition, Glycol is also regularly used to allow low temperature operation in thermal energy storage systems, where ice is made at night and used the following day to cool the building. A properly designed, commissioned and maintained glycol system can provide 20 or more years of reliable service. However, improperly commissioned and maintained glycol systems can experience severe problems in as little as one to two years. Given the high initial cost of filling a glycol loop, and the even higher cost of the resulting problems if a glycol loop goes bad, it is important to understand how to properly select and maintain a glycol system.
Ethylene vs. Propylene Glycol
Typically either ethylene glycol or propylene glycol are used in HVAC closed loops, with the type of glycol and its concentration determining the freeze point and other physical properties (see Table One). In HVAC applications, ethylene glycol is more widely used because of its lower cost, lower viscosity and better heat transfer properties. However, due to its moderate toxicity, it is subject to reporting requirements in the event of a spill or discharge (> 5,000 pounds).
Propylene glycol is much less toxic than ethylene glycol. Because it is not generally subject to reporting requirements, it is also considered more environmentally responsible. Propylene glycol is typically used in food processing facilities or other applications where there is potential for contamination of potable water or foodstuffs. Using it in a system designed for ethylene glycol can adversely affect performance. For example, the higher viscosity of propylene glycol (about 2x ethylene glycol), especially at lower temperatures, directly translates into higher pumping costs. Its poorer heat transfer properties can also reduce cooling capacity. Automotive antifreezes are unsuitable for HVAC and industrial applications because the high levels of silicate inhibitors can cause heat exchanger fouling and pump seal failures.
Preparing a System for Glycol Addition for Existing and New Systems
Existing systems should be cleaned and flushed thoroughly to remove any rust, scale and sediment before charging the system with the chosen glycol. For large systems or systems where corrosion is already evident, consult a professional industrial cleaning organization as this procedure may be involved and should be done by an experienced company. If chemical cleaning is used, it is important that all traces of the cleaning agent be removed and the system be thoroughly flushed with water.
New systems are typically coated with oil, grease or a protective film during fabrication, storage or construction. Dirt, solder flux, welding and pipe scale can also cause problems. Therefore, thorough cleaning of new systems is recommended. A solution of one to two percent trisodium phosphate can be used with water for flushing the system. Other commercially available cleaning products may also work. System volume can be calculated during this stage by metering in the initial fill of the system or by chemical analysis of cleaning chemicals aft er known quantities are introduced into the system.
Freeze Point
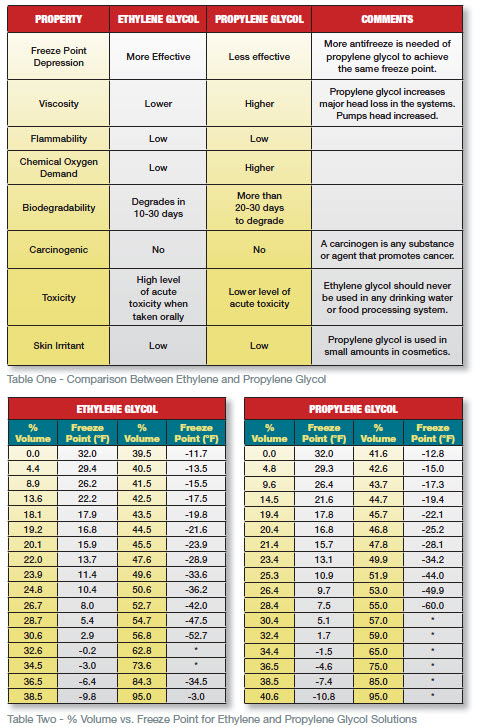
When selecting a glycol concentration based on the desired freeze protection, ensure that the freeze point is at least 5F below your lowest anticipated operating/exposure temperature (Table Two). Water Quality
High quality water should be used for glycol-water solutions. In general, the water quality should follow these guidelines, especially with higher temperatures:
- < 50 ppm calcium hardness
- < 50 ppm magnesium hardness
- < 25 ppm chloride
- < 25 ppm sulfate
If the hardness or other parameters are significantly higher than recommended guidelines, soft ened or de-ionized water should be considered.
Precautions
Automatic water-only makeup addition is generally not recommended for glycol systems, especially if used in low temperature applications (< 40oF). Any makeup addition may require that additional glycol and inhibitors be added to maintain the desired level of protection.
Inhibited vs. Uninhibited Glycol
Glycol systems will not be adequately protected using conventional closed system treatment programs. They require high levels of specific inhibitors and buffers for protection against corrosion and glycol degradation. These inhibitors can come premixed with the glycol or added as supplemental treatments. Glycol, sold with the inhibitors and buffers, included in the formulation is known as inhibited glycol. Inhibited glycols typically contain four to six percent inhibitors with the remainder being either ethylene or propylene glycol. When using inhibited glycol products, a minimum of 25 percent glycol must be used to ensure the proper inhibitor levels are achieved. If this 25 percent concentration is not required for freeze protection, three options exist:
- Add a higher concentration of the inhibited glycol than needed, which can be expensive.
- Add compatible supplemental inhibitors and buffers to raise the levels into the desired range.
- Purchase uninhibited glycol and use supplemental products to provide the necessary level of inhibitors and buffers. Depending on the cost difference between inhibited and uninhibited glycol, option three can be a cost-effective approach, especially in larger systems.
However, it is important that your water treatment supplier understands the unique treatment requirements associated with glycol systems and recommends appropriate products.
Corrosion and Degradation
Glycol degradation occurs via three modes: thermal (heat), aeration (oxygenation) and/or microbiological. As a general rule of thumb, the higher the operating temperature and the greater the aeration of the system, the greater the rate of glycol breakdown will be. The products of degradation are various organic acids and water. With respect to microbiological degradation, the system fluid is prone to biological activity if kept below 25 percent glycol. Untreated glycol solutions are extremely corrosive and will eventually degrade to form organic acids that depress the pH and further contribute to corrosion. Systems containing glycol can have serious, long-term problems, unless the proper treatment measures are taken to minimize corrosion and degradation.
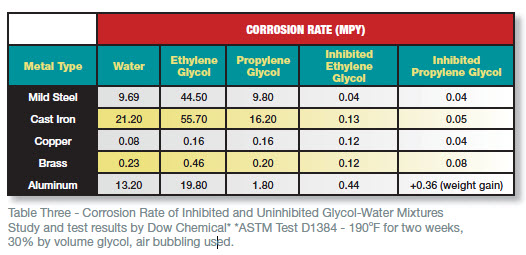
A comparison of the corrosivity of uninhibited and inhibited glycol-water solutions can be found in Table Three. Note that an uninhibited ethylene glycol solution is four-and-a-half times more corrosive towards carbon steel than plain water. If the glycol is allowed to degrade, the corrosion and associated problems will be even more severe.
An additional concern is glycol contamination. This occurs when a small amount of glycol is introduced into a system, for example the incomplete flushing of winterized coils. When glycol enters a system, bacteria use the glycol as a food source and generate acidic waste products that will rapidly reduce the pH of the loop well below the minimum of eight necessary to impede corrosion. Once the pH is reduced, it begins to solubilize old iron corrosion products, causing the water to turn black and develop a characteristic septic odor. The system is then subjected to under deposit corrosion and acidic conditions, which result in the life of the system being severely reduced.
Testing and Monitoring
Glycol-water solutions should be tested regularly to determine the percentage glycol, pH, reserve alkalinity, inhibitor levels and degree of contamination. A specially calibrated refractometer is the most reliable and practical means of determining the percentage glycol in the field. Periodic laboratory analysis of the glycol solution is recommended.
Glycol degradation is a serious problem that can be difficult to address once started. Symptoms of glycol degradation include: pH depression, presence of a sharp aldehyde or septic odor, severe steel corrosion often accompanied by high copper and iron levels, and microbiological problems. Depending on its severity, salvaging the degraded glycol may not be practical. Closed loops that contain a high-quality, properlyinhibited glycol solution will provide years of low operating maintenance, provided periodic testing and monitoring are performed.
This article was co-authored by Allan Browning, Technical Marketing Manager, Chem-Aqua and David Berry, Engineering Manager, Chem-Aqua Canada.