by Mike Morgan — Closed heating and cooling water systems are an integral part of the HVAC system in most commercial and institutional buildings. They circulate hot water to provide heating in the winter and chilled water to provide cooling in the summer. Unlike the cooling tower, no evaporation occurs in a closed loop, so there is no need for blowdown. Since the makeup water is generally potable water, some may think the closed loop can be filled with water and left alone. However, this is not a viable option.
The problems found in a recirculating cooling water system fall into the following categories:
- Corrosion
- Scale
- Fouling Deposits
- Microbiological
In closed-loop systems, scaling is not as much of a problem as in open-loop systems. However, corrosion, fouling deposits and microbiological growth have equal potential to contribute to shutdown as in evaporative systems.
|
|
It is important to remember that not all closed loops are typical chilled or hot water loops. These include:
- Thermal storage tanks that utilize thermal energy conservation for use during peak power use (thus cost) periods. Because volumes are larger than conventional closed loops, attempts to reduce treatment levels compound problems rather than prevent them. For this reason, residuals should never be reduced just because the volumes are large.
- Water systems that hold large volumes of water and remain idle for significant periods of time. These often promote the three problem factors above. Examples of this type of closed system include fire water systems and “post hydrotest” water for systems intended for normal closed or even open loop use.
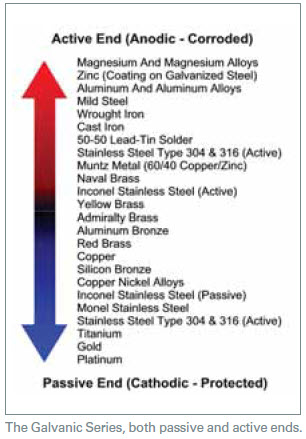
- Design configurations with a mixture of the metal piping present. Besides steel and cast iron, metals common to closed loops include brass, copper, aluminum and stainless steel. There are other metals found in lesser degrees, but these are the main mixtures. The presence of mixed metals increases the potential for galvanic-induced corrosion. Galvanized metals are normally avoided for use in closed loops due to the susceptibility to this galvanic related attack caused by dissimilar metals. (See list at right.)
|
|
Corrosion Problems
When corrosion products accumulate, deposits are formed and collect in the lower flow areas. As the morphology of these deposits becomes complex, the oxygen concentration becomes depleted under the deposit cap (tubercle). This condition promotes a differential oxygen cell leading to additional corrosion. It also creates a condition that is ideal for microbial growth.
Biologically, the growth of biofilm will insulate heat transfer and increase energy costs. In a closed loop, corrosion under these biological deposits can lead to pipe and equipment failure. As the swimming bacteria (planktonic organisms) move from one area to another, the entire system becomes inoculated with the growing biofilm (sessile bacteria). Since there is no blowdown in a closed loop, the period of time the water is held in the enclosed piping is very long. The corrosion products can build up and biological activity start to develop. As these problems develop, the situation worsens. Any scale that does form also becomes a developing problem due to hindered heat transfer.
|
|
Treatment levels should be monitored to maintain effective inhibitory ranges. Limitations of chemical to penetrate the biofilm and deposits further complicate the treatment issues. As small leaks develop (such as pump seals) treatment chemicals are lost and diluted as fresh makeup water is added. Over time, corrosion grows worse, further complicating the water loss and subsequent generated problems. Fresh water is often sanitized (such as potable water), but is very seldom sterile. It has been widely established that fresh water is the most common source of biological contamination.
Microbiocides are added to water to effectively prevent organisms from becoming a problem. It is very important to be sure to add any biocides in sufficient amounts to kill bacteria that could be a developing growth. A “sufficient” quantity is essential because a product fed below a minimum inhibitory level promotes bacterial acclimation toward the sanitizing chemical.
Microbiocides should be added based on a predetermined frequency. Reoccurring doses of biocide are required because microbiological inoculation is an ongoing process, and, over time, all biocides hydrolyze to non-hazardous byproducts to lose effectiveness. Biocides are molecules designed to lose effectiveness over time so as to not harm the environment. The “half life” of biocides is measured over hours. Soft water removes any scale potential that might be present. Scale potential will be generally associated with heat, which might come from a hot water hydronic system or a high heat flux unit normally associated with furnaces. The latter unit can be normally identifi ed with high-temperature metal-smeltingrelated processes or waste-burning fuels such as a paper mill burning black liquor. Proper chemical treatment of any closed loop should begin before it is started up as a new system. Flushing and pretreatment are necessary steps to clean and prepare the surfaces to receive corrosion inhibitors. System control can be lost long before any chilled water (or hot water) is circulated. This is due to incomplete pretreatment, possibly as a result of reducing the pretreatment programs to save time. This is a mistake that can become costly, but the net effect is never 100% visible. As an example, see the photo above. The center and right pipes show incomplete pretreatment and passivation. Preferred is the pipe at left.
|
|
Closed-loop inhibitor chemicals often involve blended corrosion inhibitors. Typical inhibitors might contain a blend of nitrite and borate (or other buffering agents), plus a copper corrosion inhibitor. Also found in many formulations are dispersants and organic scale inhibitors. Silicate is often added as protection for any aluminum present. The presence of aluminum will probably require some additional techniques for total protection, such as pH targets. Molybdate is a popular alternative. With somewhat localized geographical attempts to limit nitrite and molybdate use, organic-based inhibitors are becoming popular. An approach many suppliers offer is to provide the blended products with a color-indicating dye. The dye is effective for observing small leaks that may promote the problems present. However, caution should be in the offering if the indicating dye is used as a control method for chemical residual based on color intensity. The dyes used are often more persistent compounds than the inhibitors doing the real work. If indicating dyes are used as a control, they should not be a total replacement test for the inhibitor levels.
|
|
Filter Sizing and Selection
Removal of suspended foulants can be accomplished with filtration. Since filter backwash would be a source of fugitive water losses, a cartridge-insertion-type filter is most common for closed loops. Selecting the proper filter mesh size is a critical engineering step. When the opening is too small, the filter will plug too quickly and a high rate of maintenance is required for replacement. When the filter is too large, the particulate matter is not removed.
A common practice is to start with a larger micron size (for example 50u) and gradually work down to a small mesh size not requiring maintenance. This is usually a 5-10u filter unit. For reference, the human eye can only differentiate particles down to about 40u. Sizing a filter can be a program-saving decision. Bigger is usually better, but not everyone has an unlimited budget. A nominal starting point for economic operation is to filter about 2-5% of pumping capacity and capacity sufficient for one volume turnover for each one to three days.
Acceptable limits have been established for a well-controlled closed loop, regardless of the type. First, microbiological counts should be held to a maximum of 103 cfu/ml of aerobic activity. Anaerobic activity should be held at zero. Some observers accept one or even 10 cfu/ml for anaerobic activity because the number appears low. However, if one or 10 cfu are identified in the bulk water, there must be biofilm growing and hiding somewhere in the system. This can be the case, even if aerobic counts are =103 cfu/ml. Biological targets are intentionally held at lower limits than in open, recirculating systems. The lower counts are due to the long holding times involved. Corrosion coupons are recommended for monitoring the effectiveness of corrosioninhibiting- treatment chemicals. Typically, 90-day coupons are used for demonstration of program effectiveness. Coupons should be exposed to water velocities representing system conditions, usually three to five feet/second. To calculate velocity in ft/sec, the expression equals gpm through the corrosion coupon rack divided by 7.48 and by 60, then divided by the coupon rack piping cross-sectional area in ft2.
|
|
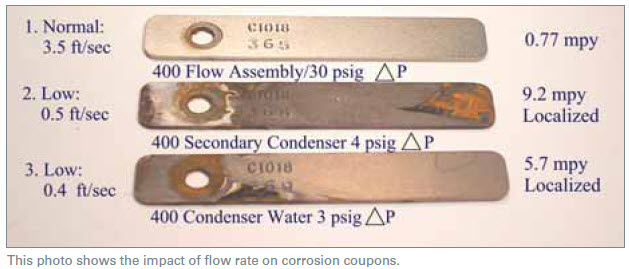
The most reliable method of insuring representative velocity should be with a direct readout flowmeter reading gpm. Flexible orifice-type flow-throttling devices should be used with caution. These “valves” depend on a pressure differential that can exceed those normally found. Typical requirements are 30 psig to insure seven gpm through a one-inch coupon rack. A Dole Valve is an example of this flexible-orifice valve.
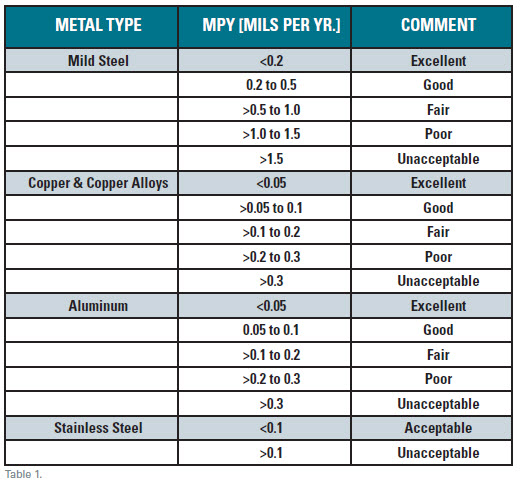
System-conditions and exposure for the coupons shown in the photo above were identical, except for the monitored flow rates. A typical assessment for program effectiveness (closed loops only) is shown in Table 1. Any pitting-type corrosion is unacceptable due to localized attack being extremely concentrated. Even at an acceptable mpy (mils per year) penetration, a localized rate failure may be close at hand due to the localized concentration of attack.
Mike Morgan is senior corporate engineer for
Chem-Aqua (www.chemaqua.com), a global water
treatment solutions provider since the 1920s. The
company designs custom programs that solve water
treatment problems and make boiler, cooling, and
process water systems more efficient.